Drug developers and regulators are expanding the use of real world evidence to design, test and review rare disease treatments. But market access challenges persist as payers in the U.S. and Europe continue to express doubts. A new survey sheds light on the perception gap.
A striking life sciences trend has come into focus the past 12 months. Global regulators—often viewed as taking a conservative approach to transformative technology—have moved into the vanguard in discussions and guidance around real world data (RWD) and real world evidence (RWE).
The shift is unfolding with uncharacteristic speed. In December 2018, the U.S. Food and Drug Administration unveiled a Strategic Framework on RWE, accompanied by detailed commentary by agency leaders. Five months later, the FDA expanded the guidance and created a unified format for submitting new drug and biologic applications involving RWE. In invited comments, industry organizations such as BIO and PhRMA urged the FDA to increase manpower for accelerated reviews where RWE comes into play. Within months, and perhaps not coincidentally, the agency restructured its Office of New Drugs to do exactly that.
“The reorganization will help the agency identify resources for more training of divisional review staff in new techniques for regulatory science including the use of RWE,” says Peter Pitts, former FDA associate commissioner and consultant for Syneos Health.
In some cases, biopharma companies working in rare diseases helped pioneer the very approaches regulators discuss in the latest guidance. Many are aligned with its roadmap. But the same cannot be said for all stakeholders. To date, market access for new medicines emerging through pathways involving RWE has not kept pace.
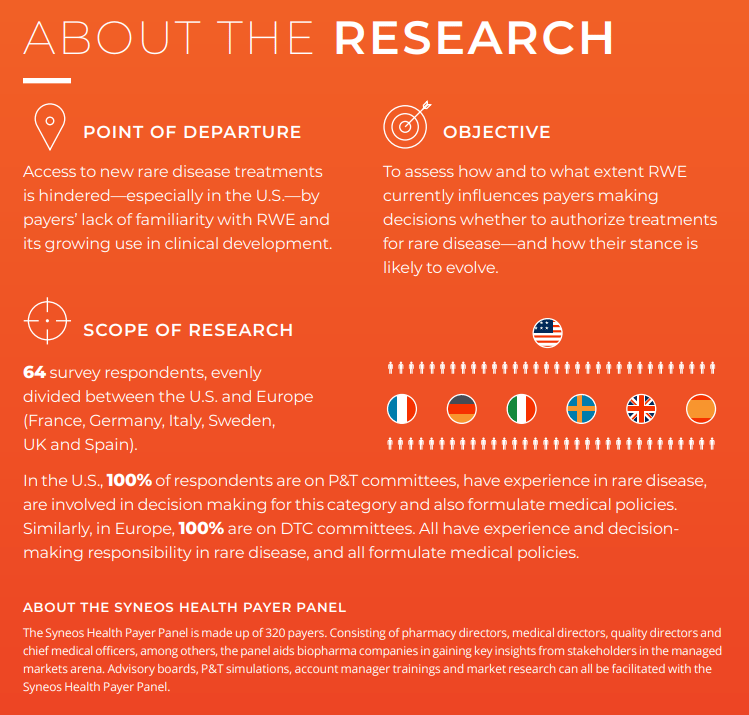
In both Europe and the U.S., payer organizations have embraced RWE in coverage decisions on rare disease treatments only within limited parameters. According to new research by Syneos Health on payer perceptions of RWE in rare diseases, many payers are not entirely receptive to RWE-based representations or health economic forecasts in sponsors’ dossiers or related conversations.
What accounts for payers’ hesitation? Partly, it’s because they are focused on conditions such as cancer, diabetes and cardiovascular disease that are a much larger piece of pharmacy and medical budgets. Lack of urgency means there’s little incentive to learn the terminology of RWE. In addition, those who are familiar often express deep concerns about data quality and the potential for sponsor bias.
Many payers are not entirely receptive to RWE-based representations or health economic forecasts in sponsors’ dossiers or related conversations.
The following discussion will examine these issues from several perspectives based on conversations with payers, drug developers, regulatory experts and patient organizations. We also will summarize and expand on these stakeholders’ best-practice recommendations.
Together—and sooner rather than later—concerned parties from all quarters must clarify appropriate pathways for generating and using RWE. This is the only way to increase responsiveness of payers who are the gatekeepers to market access for patients struggling with difficult-to-treat conditions.
Perception Gaps on Real World Evidence in the Rare Disease Space.png